Frequently asked questions
In this section, we provide basic information on what clinical trials are and how they may be accessed. More information about clinical trials in Canada (in oncology and beyond) can also be found on www.itstartswithme.ca
Please speak to your treating oncologist for specific questions.
How does the clinical trial database work?
The U-Link.care clinical trial database provides information about phase 1 and 2 clinical trials in pediatric haematology and oncology. Phase 1 and 2 studies will be implemented first, with the aim to eventually encompass all studies, including phase 3.
Why does the U-Link.care database only offer information about trials available in Canada?
The U-Link.care website aims to provide the Canadian pediatric haematology oncology community with a tool, allowing care teams to determine the availability and status of clinical trials for their patients. By focusing on Canada, we make sure we are offering a very responsive tool because we have the capacity to keep it up to date.
I am a patient or parent of a child with cancer, how can I participate in a clinical trial?
To enroll on a clinical trial, a patient with cancer must meet the particular eligibility criteria for the trial. Each clinical trial will have its own unique set of criteria. For example, a patient may need to be diagnosed with a particular type of cancer, may need to have banked stem cells, or may need to have completed their past treatment within a certain time period. These eligibility criteria can be confusing.
Your care team will work carefully with you to determine if the clinical trial is a good fit for you or your child and if you meet the particular requirements to enroll on the trial.
Please talk to your care team about these requirements if you see a clinical trial of interest.
I am a health care provider, how can my patient access a clinical trial?
Once the eligibility criteria have been verified (inclusion criteria and non-inclusion criteria), the patient is then eligible for a clinical trial. Only study investigators may enroll the patient on a clinical trial. In addition, only open centers for a given trial can take care of a patient enrolled on the trial so it is necessary to refer the patient to an investigator in the relevant centre.
What benefits are there for a child participating in a clinical trial?
A child participating in a clinical trial
- will have access to innovative therapies,
- will receive intensive follow-up during and after treatment,
- will receive supportive care for all aspects of treatment.
When you are enrolled on a clinical trial, the patient and/or their family can decide to stop participation in the clinical trial at any point. This will not jeopardize the care that the child receives, and they will continue to receive care and specific follow-up. In addition, the child’s care team can decide to stop the trial at any time if they consider the new treatment to be less beneficial than existing treatments. The care team will always share their concerns with you before stopping the trial.
What benefits and risks are there for a child participating in a clinical trial?
When you or your child are enrolled on a clinical trial, good things can happen. These are called “benefits”. A benefit of a clinical trial is that the new treatment will stop the cancer from growing or make it go away all together. Another benefit is that the information learned about new treatments in a clinical trial can help other cancer patients in the future.
When you or your child are enrolled on a clinical trial, bad things can happen too. These are called “risks”. Risks may include losing your hair, having to stay in the hospital for longer periods of time, and receiving medicines that might make you feel sick. There are also risks that the treatment may not make your cancer go away and even risks that your care team does not know about yet. When you or your child are enrolled on a clinical trial, it is important to tell your care team if you are feeling different or sick in other ways than you have experienced before. Your care team will always try to make you feel better and may give you other medicines to help with how you are feeling.
Many treatments in childhood cancer clinical trials have already been studied with adults where they have learned about many of the possible side-effects. These side-effects are listed in the consent form that your care team will take you through and that you will sign. Side-effects from treatment often vary from patient to patient so it may not be possible to know what side-effects a patient will experience before the treatment starts.
How are the child and their family informed about the benefits and risks of the trial?
Before you decide to enroll on a clinical trial, your care team will go through the details of the trial with you. You will be able to ask any questions about the information that was shared with you. If you do not understand a detail about the clinical trial, we encourage you to ask your care team so that you are comfortable with all the details of the study.
When you are ready, you will be asked to sign the consent form. The consent form is not a contract, but your signature is necessary before starting any treatment. Signing the consent form does not mean that you have to stay on the clinical trial. If you feel uncomfortable at any time during the clinical trial, please speak to a member of your care team.
The consent form will cover many different topics.
Some of these include:
- possible benefits for the patient
- known risks and side effects,
- duration of study,
- a description of the treatment,
- your rights as a participant
How do I talk to my child about clinical trials?
How do I talk to my child about clinical trials?
Explaining cancer clinical trials and treatments to your child can be challenging. You will know best as a parent or caregiver in how to talk to your child about everything they are experiencing and explaining concepts in a way that they will understand. If you would like help talking to your child about clinical trials, please reach out to your care team.
You could also share an animation called “The Participators” with your child. This animation was created by kids, for kids and explains some of the important concepts around clinical trials. https://youtu.be/fFQ8FvSMezo
Once a child or adolescent is enrolled in study, can parents or the patient change their mind?
Whatever the situation, the parents of a child participating in a clinical trial may, at any time, decide to no longer participate. The child will continue to receive support and specific follow-up from the care team.
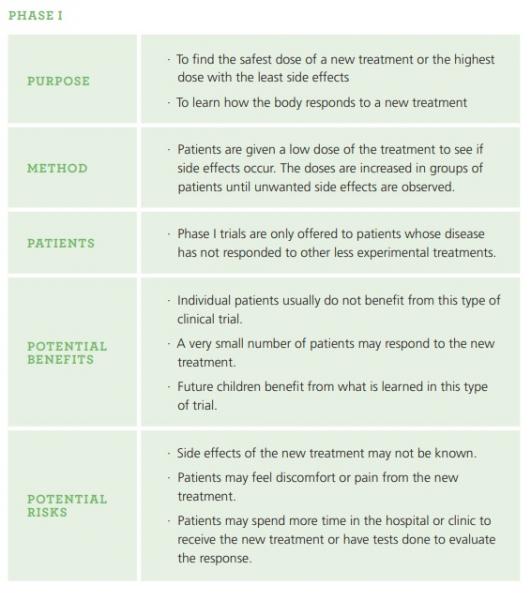
What are the different phases of clinical trials? (information from itstartswithme.ca and childrensoncologygroup.org )
Clinical trials involving new medications are done in a series of steps called phases so researchers can learn about new medications in a gradual and safe way. Phase 1 is generally the first time these medications are given to people. Participants are closely monitored throughout each phase. Information and results from one phase are used to help design the next phase and the clinical trial only moves on to the next phase when the previous phase’s results were considered to be positive.
In addition to the phase 1, 2 and 3 clinical trials, you may also hear about pilot studies. Pilot studies are small scale studies done to see if a new treatment or research method will be useful in a larger scale and usually happen before or in lieu of a phase 1 trial.