Go to family friendly version
| Diagnosis | Relapsed/Refractory Solid tumour (+ lymphoma) or CNS tumour | Study Status | Open |
| Phase | I/II |
| Age | 0 Years to 21 Years | Randomisation | NO |
| Line of treatment | Disease relapse or progression |
| Routes of Treatment Administration | Arm A | Drug: Irinotecan (IV), Temozolomide (Oral), Paxalisib (Oral)
|
| Last Posted Update | 2026-01-08 |
| ClinicalTrials.gov # | NCT06208657 |
International Sponsor
Australian & New Zealand Children's Haematology/Oncology GroupPrincipal Investigators for Canadian Sites
The Hospital for Sick Children - Dr. Daniel Morgenstern
CHU Sainte. Justine - Dr. Monia Marzouki
BC Children's Hospital - Dr. Rebecca DeyellCentres
Medical contact
Dr. Henrique Bittencourt
Dr. Monia Marzouki
Dr. Sebastien Perreault (neuro-onc)
Social worker/patient navigator contact
Marie-Claude Charrette
Clinical research contact
Marie Saint-Jacques
Medical contact
Rebecca Deyell
Social worker/patient navigator contact
Ilana Katz
Clinical research contact
Hem/Onc/BMT Clinical Trials Unit
Study Description

 This study is eligible for STEP-1 funding. Find more information here.
This study is eligible for STEP-1 funding. Find more information here.
Both Australia (Zero Childhood Cancer) and Canada (PROFYLE) have developed precision oncology programs for the pediatric population through which samples from childhood/adolescent cancers undergo in depth genetic profiling. OPTIMISE is a companion platform trial, which will link patients to novel targeted agents based on their tumor profile. The trial will have multiple basket arms based on the most common genetically altered pathways the investigators have identified in these childhood cancers. Each arm of the trial will be histopathology agnostic and test a rational, novel combination therapy, to maximise potential clinical benefit.
The information below is for Treatment Arm A which will be combining, for the first time, paxalisib with conventional chemotherapy in paediatric patients with high-risk malignancies.
Inclusion Criteria
- Patients must be diagnosed with a solid tumor, CNS tumor or lymphoma that has progressed despite standard therapy, or for which no effective standard therapy exists.
- Age <21 years at inclusion; patients 21 years and older may be included after approval by the Study Chair if they have a pediatric type recurrent/refractory malignancy.
- Patients must be enrolled on a precision medicine study (i.e. PROFYLE, ZERO or equivalent as agreed with Study Chair.
- Tumour profiling should be performed as close to the time of study enrolment as possible; at a minimum profiling should have been performed on a sample obtained within 12 months prior to enrolment, or had confirmation that the targeted molecular aberration is still present from a tumour sample collected within the 12 months prior to enrolment. Patients for whom tumour profiling has been performed outside this window may only be enrolled after approval by the Study Chairs.
- Patients are eligible to enrol using existing sequencing results or other criteria such as immunohistochemistry (provided a report from a CLIA‐approved or equivalent laboratory is provided), but concurrent enrolment on a precision medicine study is still required.
- Patients enrolled in a Phase I cohort must have either evaluable or measurable disease.
- Patients enrolled in a Phase II cohort must have measurable disease. Evaluable and measurable disease are defined by standard imaging criteria (RECIST V1.1, RAPNO or RANO) for the patient's tumor type.
- Disease evaluations, laboratory tests, and other clinical assessments that are considered standard of care may be undertaken at the patient's local oncology treatment centre with results transferred to study site for evaluation.
- Performance status: Karnofsky performance status (for patients > 16 years of age) or Lansky play score (for patients ≤ 16 years of age) ≥ 50%.
- Life expectancy ≥ 6 weeks.
- Patients must have fully recovered from the acute toxic effects of all prior anticancer therapy and must meet the following minimum duration from prior anticancer-directed therapy prior to enrolment.
- Adequate organ function.
- Haematologic criteria:
- Peripheral absolute neutrophil count (ANC) ≥1.0 x 109/L (unsupported) (i.e. at least 7 days post filgrastim; at least 14 days post PEG‐filgrastim (if administered)).
- Platelet count ≥75 x 109/L (unsupported; defined as no platelet transfusions within prior 7 days).
- Haemoglobin ≥80 g/L (transfusion is allowed).
- Renal and hepatic function:
- Serum creatinine ≤1.5 x upper limit of normal (ULN) for age
- Total bilirubin ≤1.5 x ULN
- Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) ≤5 x ULN except in patients with documented tumour involvement of the liver who must have AST and ALT ≤10 x ULN
- Able to comply with scheduled follow-up and with management of toxicity.
- Females of childbearing potential must have a negative serum or urine pregnancy test.
- Fertile males must agree to use adequate contraception during the study and following completion of treatment.
- Provide a signed and dated informed consent form.
Additional Inclusion Criteria for Arm A
- For Cohort A1: Patients must have evaluable or measurable disease.
- For Cohort A2, Molecularly selected: Tumour must have a demonstrated pathogenic/likely pathogenic mutation in the PI3K/AKT/mTOR pathway from tumour DNA analysis, including:
- Loss of function (LoF) in tumour suppressor genes or genes that normally act to downregulate the PI3K/AKT/mTOR pathway (PTEN, PIK3R1, TSC1, TSC2, DEPTOR)
[Rationale: LoF variants involving these genes can lead to increased signalling of the PI3K/AKT/mTOR pathway. This includes single nucleotide variants (SNV), copy number
loss and structural variants (SV) leading to disruption of normal gene function.]
- Gain of function (GoF) variants in oncogenes or genes that normally lead to upregulation of the PI3K/AKT/mTOR pathway.
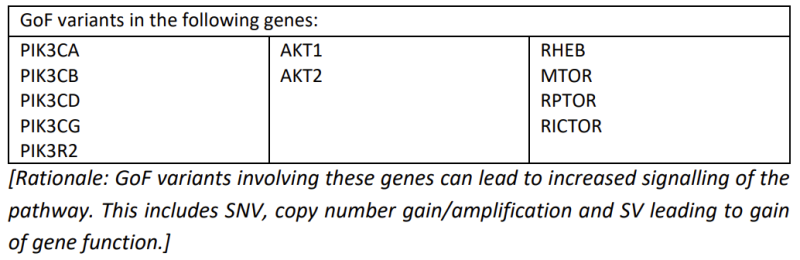
- Cohort A3, Expanded drug-selection: Patients will be eligible if they have a demonstrated PI3K/AKT/mTOR pathway aberration in tumour from RNA expression analysis or proteomic analysis which would be predicted to benefit from PI3K/AKT/mTOR inhibition after discussion with the Arm A Study Chair, or demonstrated individual tumour sensitivity to a drug acting on this pathway through pre-clinical studies (in vitro and/or in vivo studies, which may include high-throughput drug screen and/or patient derived xenografts (PDX) and/or neurospheres). Patients with pathogenic/likely pathogenic mutation in relevant genes not meeting criteria for Cohort A2 will also be eligible.
- For Cohorts A2 and A3: Measurable disease as per RECIST, RAPNO or RECIL criteria. Patients with neuroblastoma will also be eligible if they have disease that is only evaluable by MIBG.
- For all Cohorts: Adequate cardiac function defined as
- Shortening fraction of ≥27% by echocardiogram, or ejection fraction of ≥50% by gated
radionuclide study (MUGA); and
- QTC <480 msec by the Fridericia formula.
Other inclusion or exclusion criteria may apply.
Exclusion Criteria
- Patients with symptomatic CNS primary or metastatic tumors who are neurologically unstable or require increasing doses of corticosteroids or local CNS-directed therapy to control their CNS disease.
- Impairment of gastrointestinal (GI) function or GI disease that may significantly alter drug absorption of oral drugs.
- Clinically significant, uncontrolled heart disease (including history of any cardiac arrhythmias, e.g., ventricular, supraventricular, nodal arrhythmias, or conduction abnormality), unstable ischemia, congestive heart failure within 12 months of screening.
- Known active viral hepatitis or human immunodeficiency virus (HIV) infection or any other uncontrolled infection.
- Presence of any ≥Grade 2 treatment-related toxicity with the exception of alopecia, ototoxicity, lymphopenia or clinically stable peripheral neuropathy
- Major surgery within 21 days of the first dose of investigational drug.
- Known hypersensitivity to any study drug or component of the formulation.
- Pregnant or nursing (lactating) females.
- Any other concomitant serious medical condition or organ dysfunction that in the opinion of the investigator would either compromise patient safety or interfere with the evaluation of the safety of the investigational drugs.
Additional Exclusion Criteria for Arm A:
Exclusion criteria for all Cohorts:
- Patients with significant uncontrolled hyperglycaemia that in the opinion of the investigator would compromise patient safety.
- Diabetic participants who require insulin therapy.
- Patients with active pneumonitis that is clinically symptomatic.
- Patients with a history of myocardial infarction or coronary artery disease
Other inclusion or exclusion criteria may apply.